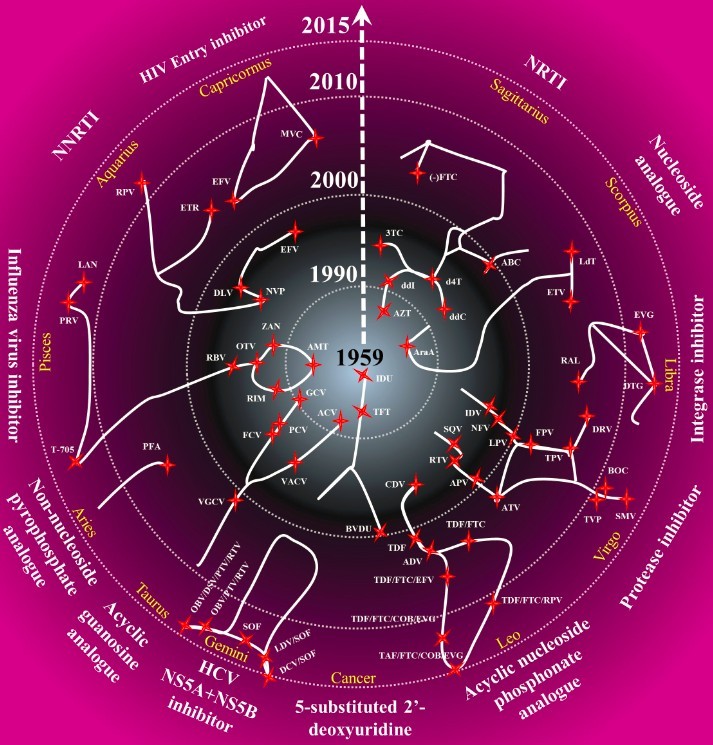
Approved antiviral drugs over 5 decades
Antiviral drug history
As of December 2015, 87 antiviral drugs have been approved for clinical use. Please click the following picture for the information of individual drugs.
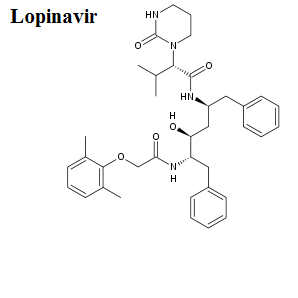
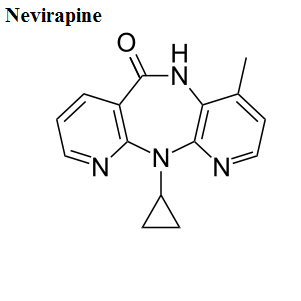
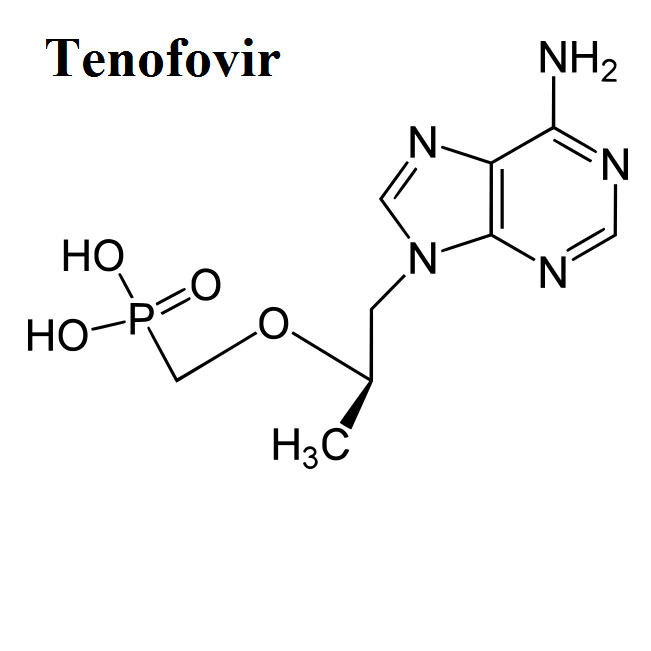
Mechanisms of drug action
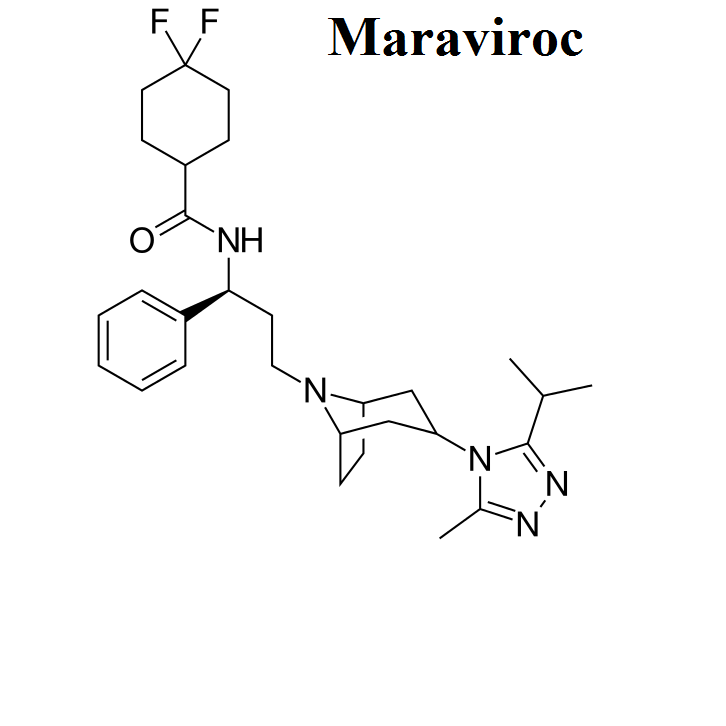
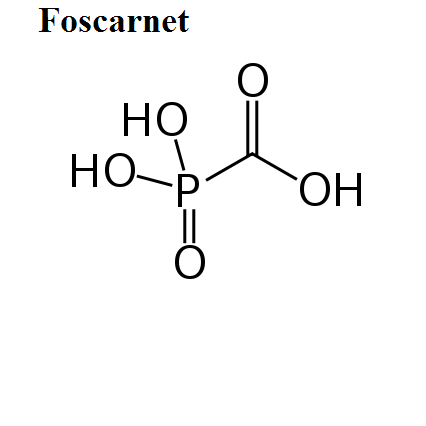
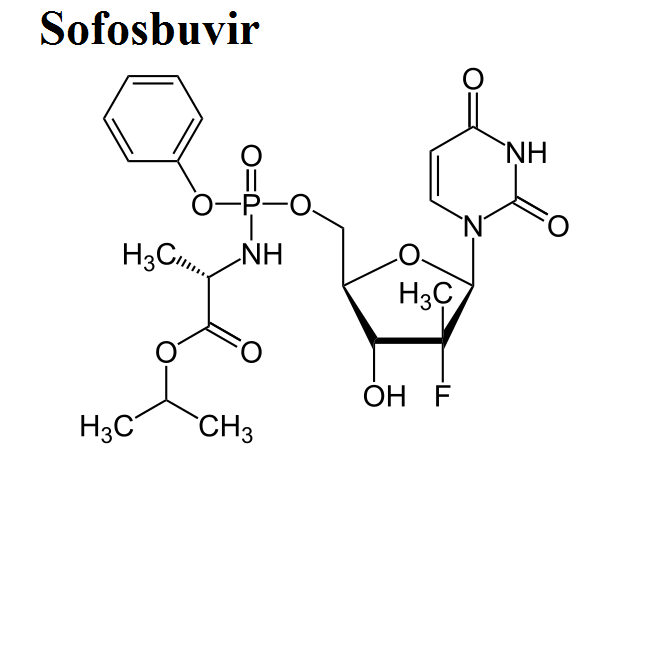
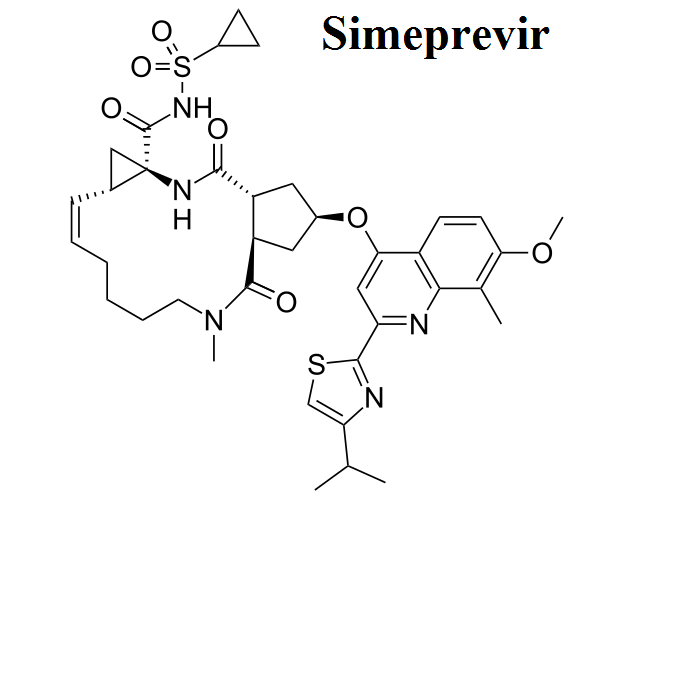
Here, we share the movies to show how antiviral drugs bind to their protein targets. 13 movies are shown for visualizing the mechanisms of drug actions.See quick selections on left side bar.
If your computer is too slow to open this page, please click websites below.
- Lopinavir
- Nevirapine
- Tenofovir
- Maraviroc
- Foscarnet
- Sofosbuvir
- Simeprevir
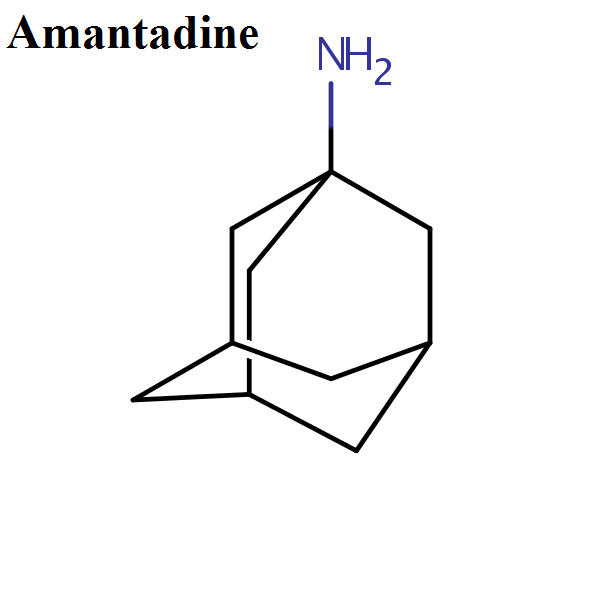
- Amantadine
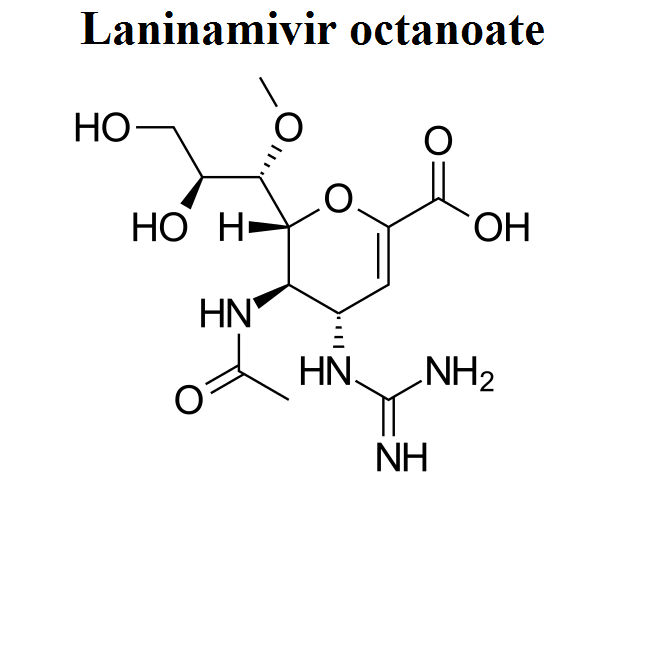
- Laninamivir
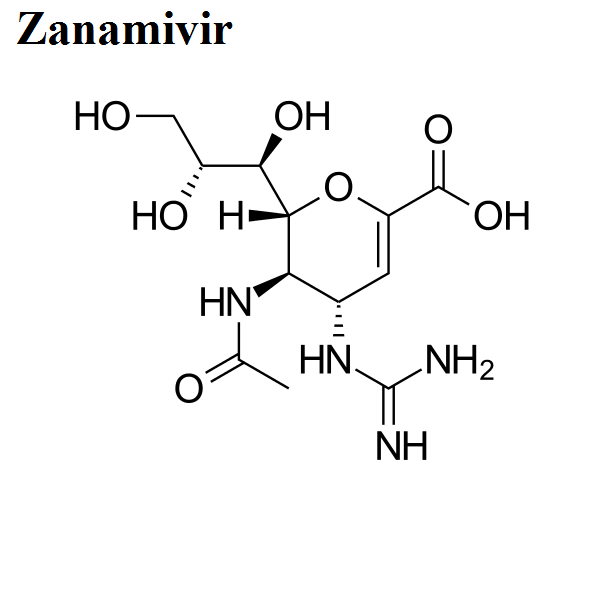
- Zanamivir
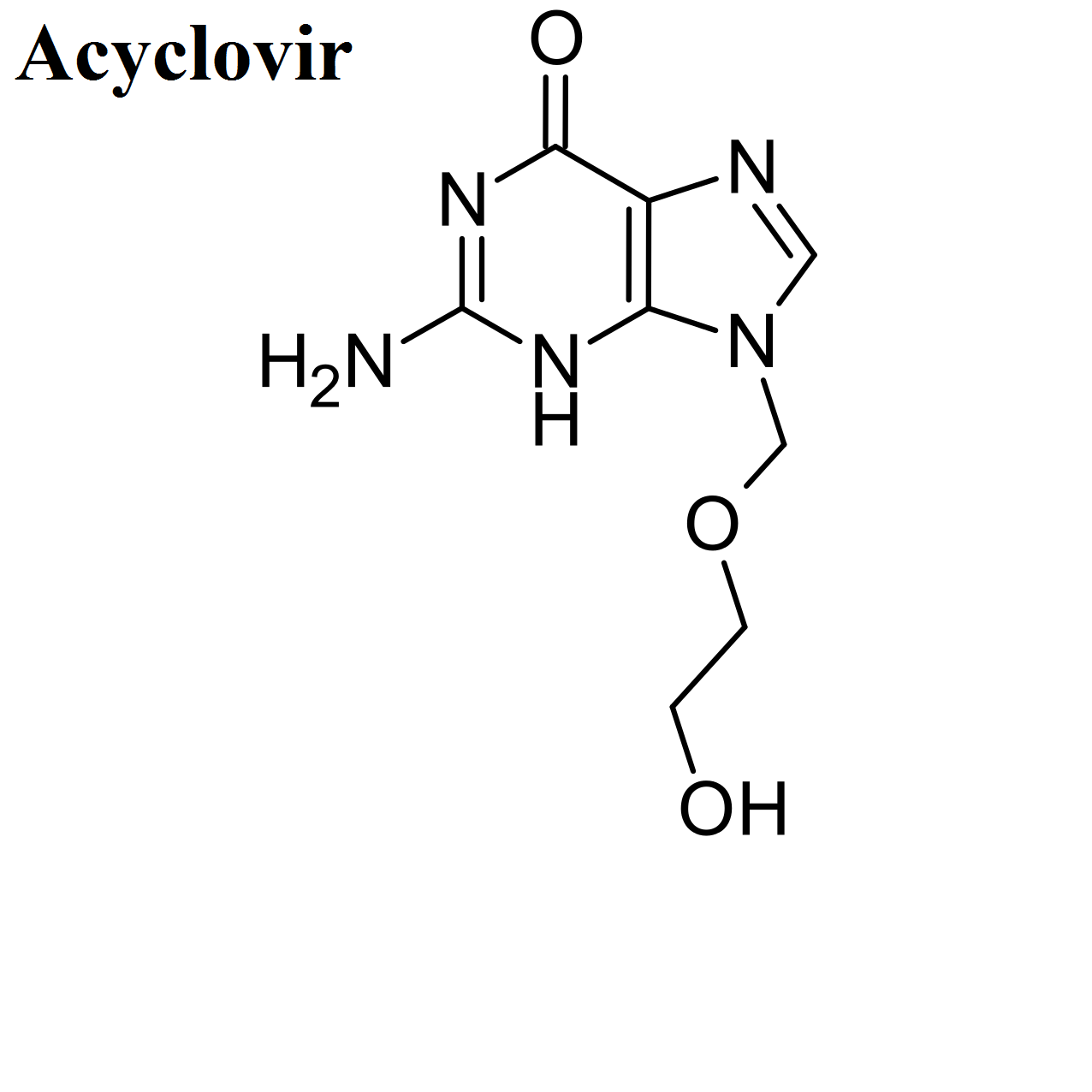
- Acyclovir
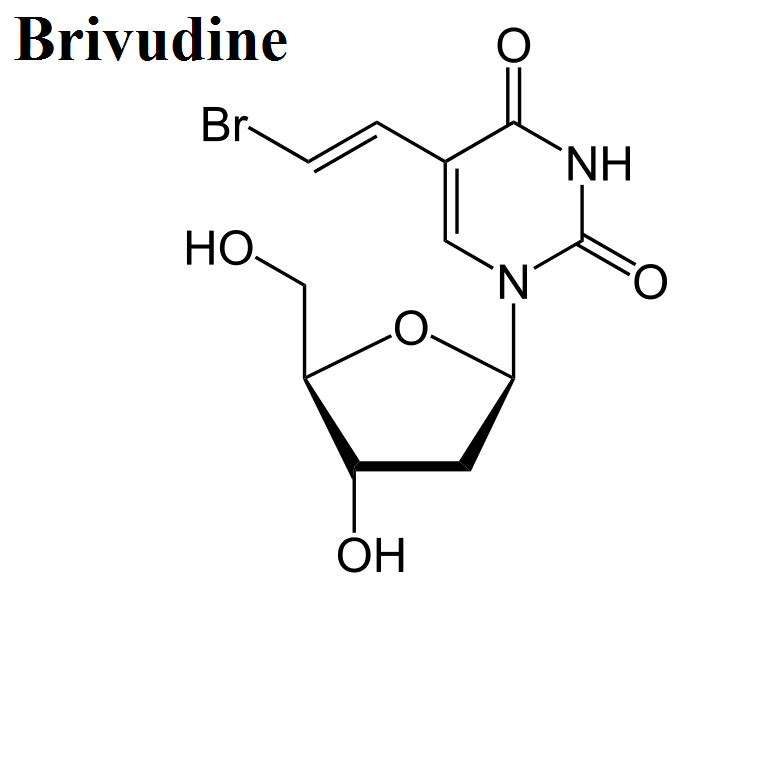
- Brivudine
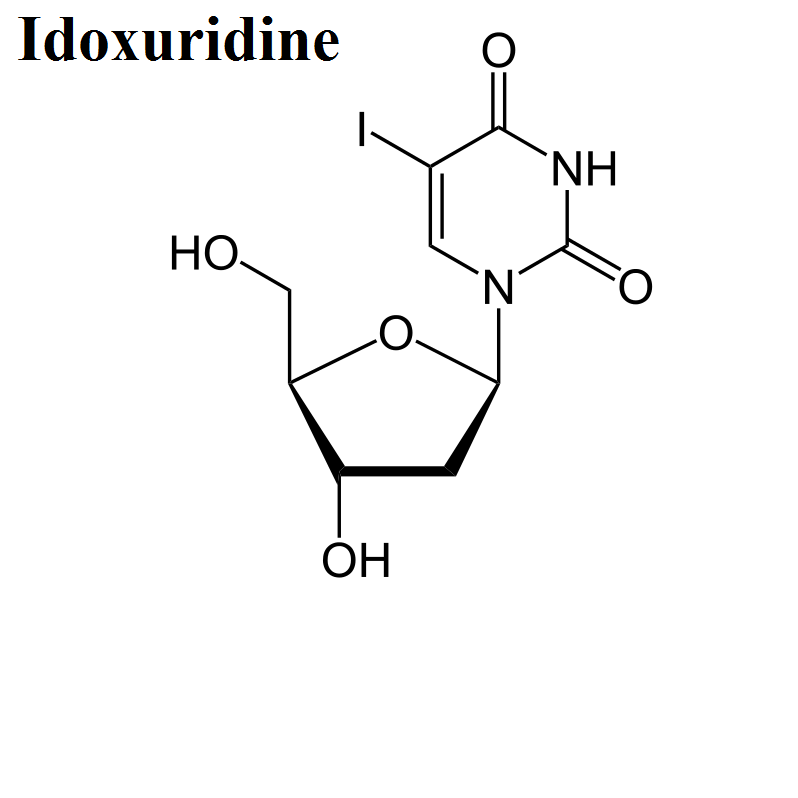
- Idoxuridine
Our team
M.D. Ph.D. Erik De Clercq
Erik De Clercq is a known physician, scientist, professor and drug developer for five decades. His landmark discoveries in anti-HIV medications include nucleotide analogues (e.g. Tenofovir), and inventions or co-inventions of several approved drugs for anti-viral therapies. Since 1967, he has published more than 2000 SCI articles. His research interests focus on many known viruses such as HIV, HCV, HBV, EBOV, poxvirus and herpesvirus.
My curriculum vitae (CV)
My Publications
Current address: Rega Institute for Medical Research, Department of Microbiology and Immunology, KU Leuven - University of Leuven, Leuven, Belgium
Ph.D. Guangdi Li
Guangdi Li obtained his Bachelor's degree in Applied Mathematics at Hunan University, China. Afterwards, he received his Master degree in Computer Science at Shandong University, China. With full scholarship, he worked as a research assistant at Universidad Politécnica de Madrid, Spain. In 2014, he obtained his Ph.D in Biomedical Sciences from Faculty of Medicine, K.U. Leuven, Belgium. During this period, he also received extensive training from Master of Molecular and Cellular Biophysics, Faculty of Science, K.U. Leuven. His research interets focus on antiviral drugs and the genome-wide diversity, coevolution and interaction of HIV, HBV and HCV.
My curriculum vitae (CV)
My Publications
Current address: Metabolic Syndrome Research Center, the Second Xiangya Hospital, Central South University, Changsha, Hunan, China
Email: liguangdi.research@gmail.com